02/05/2017
How proteins find each other to form signaling complexes
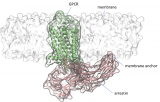
A study led by Jana Selent, head of the GPCR drug discovery group of GRIB (IMIM-UPF) and Martha Sommer (Institute of Medical Physics and Biophysics at the Faculty of Medicine in Charité Hospital, Berlin) published in the journal Nature Communications, focused on how G-protein-coupled receptors (GPCRs) and arrestin form complexes. The human GPCR family is an important class of targets for nearly half of all medicines prescribed today with the majority being involved in sensory and neuronal processes. Complex formation with intracellular signaling proteins such as arrestin is critical for many bodily processes. In this context, the published study identifies a previously unknown binding element critical to GPCR-arrestin interaction. Using a combination of computer simulations and site-directed fluorescence spectroscopy, the researchers were able to show that loops within the C-edge of arrestin are anchored to the membrane while forming pre- and high affinity complexes with GPCRs. This discovery opens up a whole new field of research regarding how the membrane influences formation of GPCR signaling complexes with arrestin. Ultimately, these insights can be exploited for proposing new strategies to modulate this important class of drug targets.
Pub Reference: Ciara C.M. Lally, Brian Bauer, Jana Selent & Martha E. Sommer. C-edge loops of arrestin function as a membrane anchor. Nature Communications, 2017. doi: 10.1038/ncomms14258.